

- Home Featured
NAFDAC Traceability: Find out all you need to know here


- Home Featured, Press Release
Relocation of Open Drug Marketers to Coordinated Wholesale Centre (CWC) in Kano: Fight Against Substandard and Falsified Medicines (SFS)

- Home Featured, Vaccine Publications
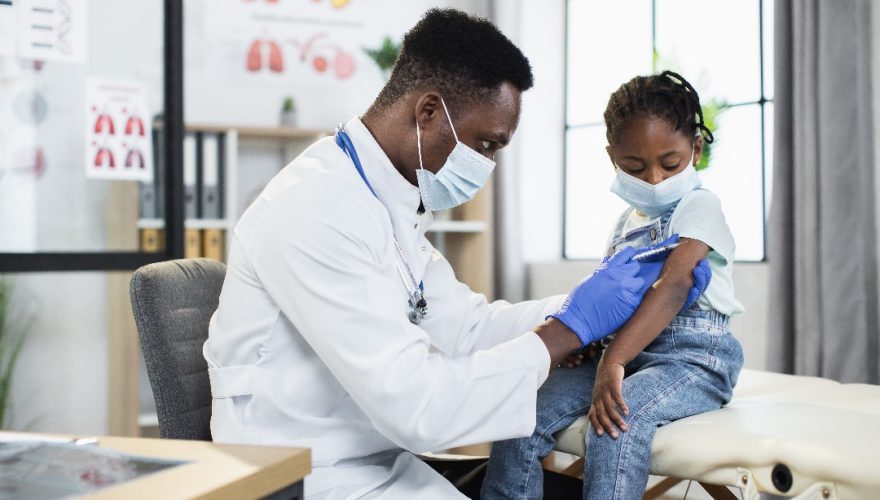
Vaccine Types And Benefits: What You Need To Know
- Home Featured, Press Release
Approval by NAFDAC on use of Gardasil Vaccine as Single Dose in the Routine Immunization
Gardasil is a vaccine that protects against human papillomavirus (HPV), a common sexually transmitted infection that can cause cervical, vaginal, vulvar, and other cancers.
NAFDAC Updates
Edit Content
Edit Content
October 26, 2023
Keynote Speech by Prof. Mojisola Christiana Adeyeye on Fighting the
Edit Content
November 21, 2022
ICMRA Communication Campaign: World Antimicrobial Awareness Week 2022
November 18, 2021
World Antimicrobial Awareness Week
Recalls & Safety Alerts
The list below are recalls and alert released by NAFDAC. The alert notices and safety communication contain safety measures to be taken and information that may impact both treatment and diagnostic choices for healthcare providers and patients.
Our Services
Edit Content
Meet the Director General.
Prof Moji Adeyeye is the Director General of National Agency for Food and Drug Administration and Control (NAFDAC) where she has added strong governance structure, regulatory system strengthening, and strengthening of the local pharmaceutical companies through international best practices with emphasis on local manufacturing.

Prof. Moji Christianah Adeyeye
Director General, NAFDAC













